EMA Approves Tx for Psoriasis and Other Autoimmune Disorders
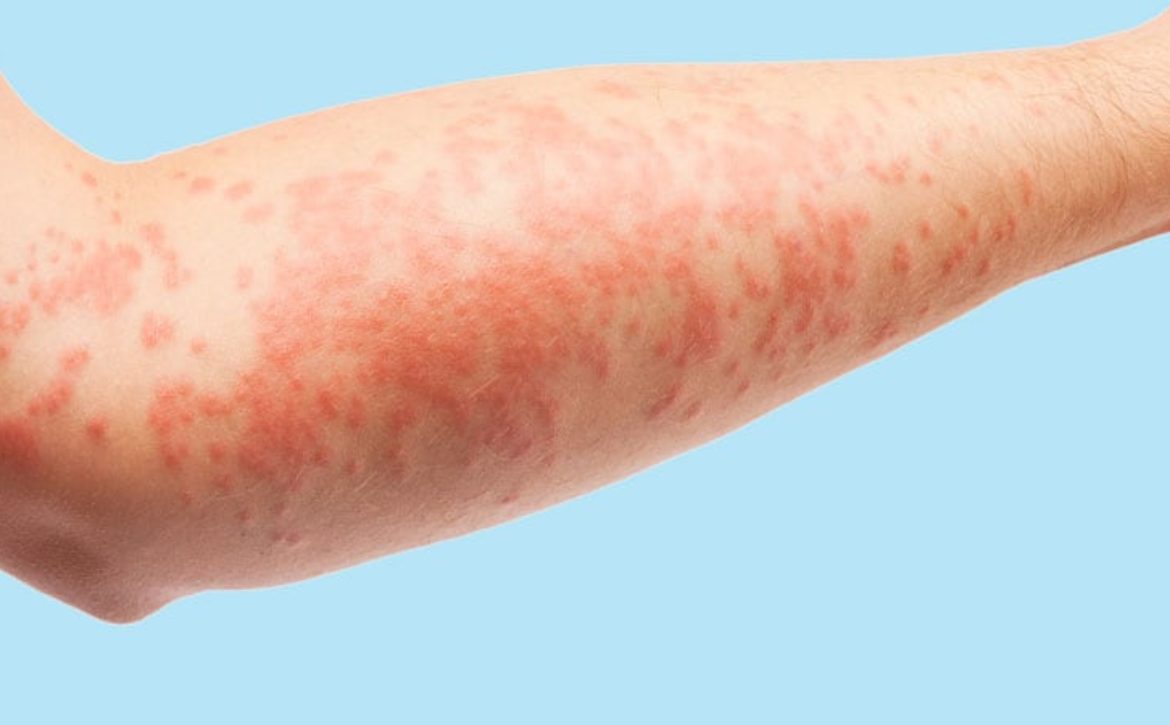
In its February 2024 meeting, the European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use has granted marketing authorizations for Pyzchiva (ustekinumab) for the treatment of plaque psoriasis, including pediatric plaque psoriasis; psoriatic arthritis; ulcerative colitis; and Crohn’s disease. It also approved Apremilast Accord (apremilast) for the treatment of psoriatic arthritis…
Read More
0